Successful gene therapy programs require large quantities of high-quality vector which traditional manufacturing has been unable to consistently provide due to instability.
See how iVexSol
Intelligent Vector SolutionsTM can solve for:
iVexSol Intelligent ManufacturingTM lentiviral vector technology stably integrates our custom DNA elements, enabling the generation of a monoclonal population of highly productive vector-producing cells. Our solutions include both stable packaging cell lines (currently available through an Early Access program) and stable producer cell lines.
How does it work?
Vector Fitness Testing
Our first step is to perform iVexSol Vector Fitness TestingTM on your current transient production system to ensure that it is fit for purpose.
Next, we use our iVexSol Intelligent ManufacturingTM to generate a monoclonal stable cell line using your gene of interest.
We then create a master cell bank of highly productive vector-producing cells.
Construct Engineering
Constructs are re-engineered for stable expression


Cell Line Engineering
Serum-free suspension cells are engineered to produce vector


Selection
Cells are seeded, expanded and screened to identify highly productive clones


Optimal Producer
Optimal producers are further screened for stability, functionality and safety


MCB Generation
Optimal clones are expanded to create a Master Cell Bank


Long Term Storage
MCB is cryopreserved and split 50/50 for off-site storage
At the completion of this process, we will have generated and archived a master cell bank of stable producer cells for your exclusive use.

The cell line engineering process is only performed once per gene of interest.
From this point on, expensive transfection reagents and plasmids are no longer required.
Following MCB generation we begin vector production and packaging by thawing a vial from your bank:
Thaw

Expand
Induce

Harvest

Fill/Finish

Release

Ship To You
How does it impact my program?
Our Intelligent Manufacturing TM ensures the rapid and reliable production of high quality, high-titer, “ready to use” vector on time and at any scale, accelerating your clinical development timeline.
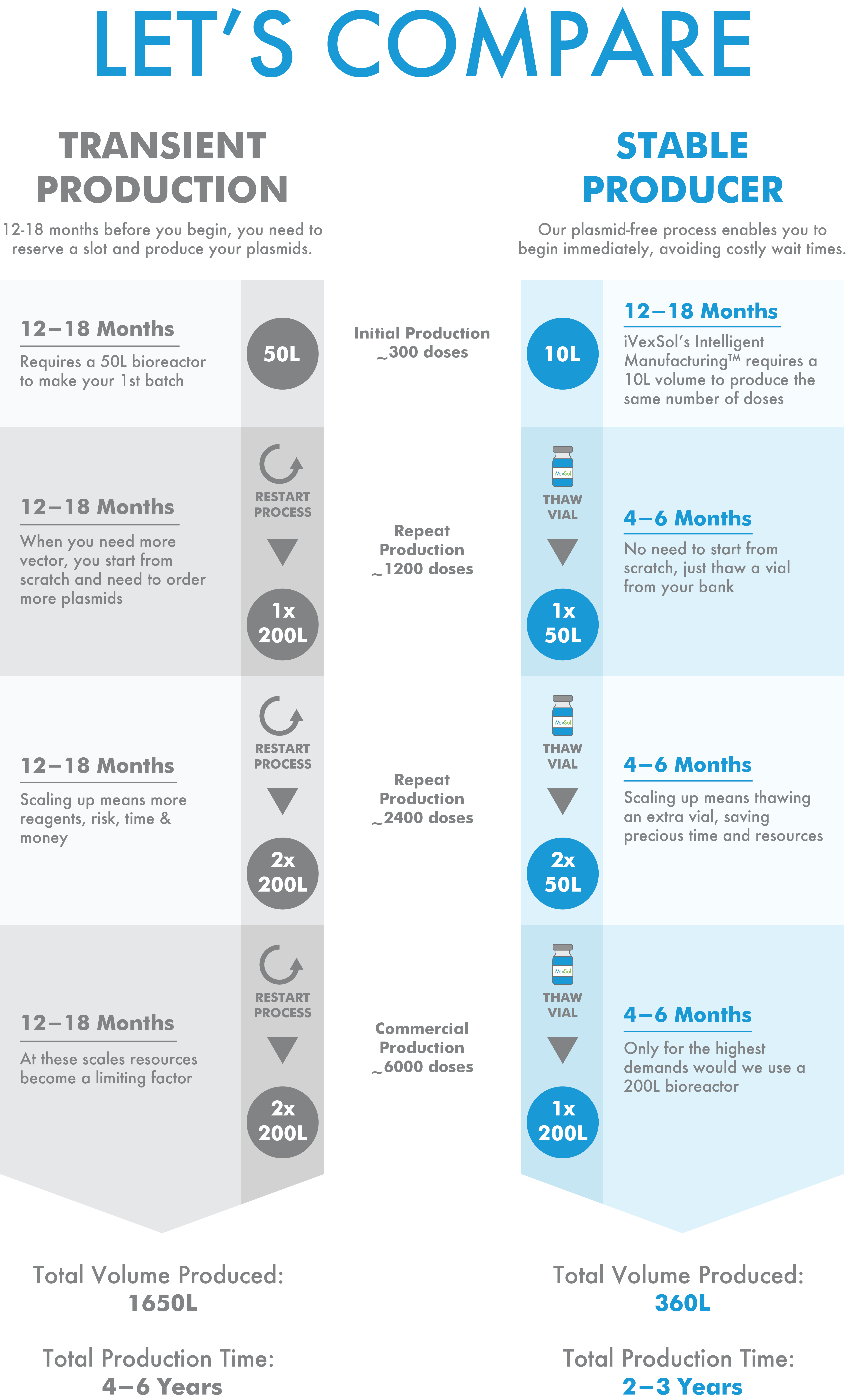
Our highly scalable platform supports your vector needs through drug development and commercial launch.
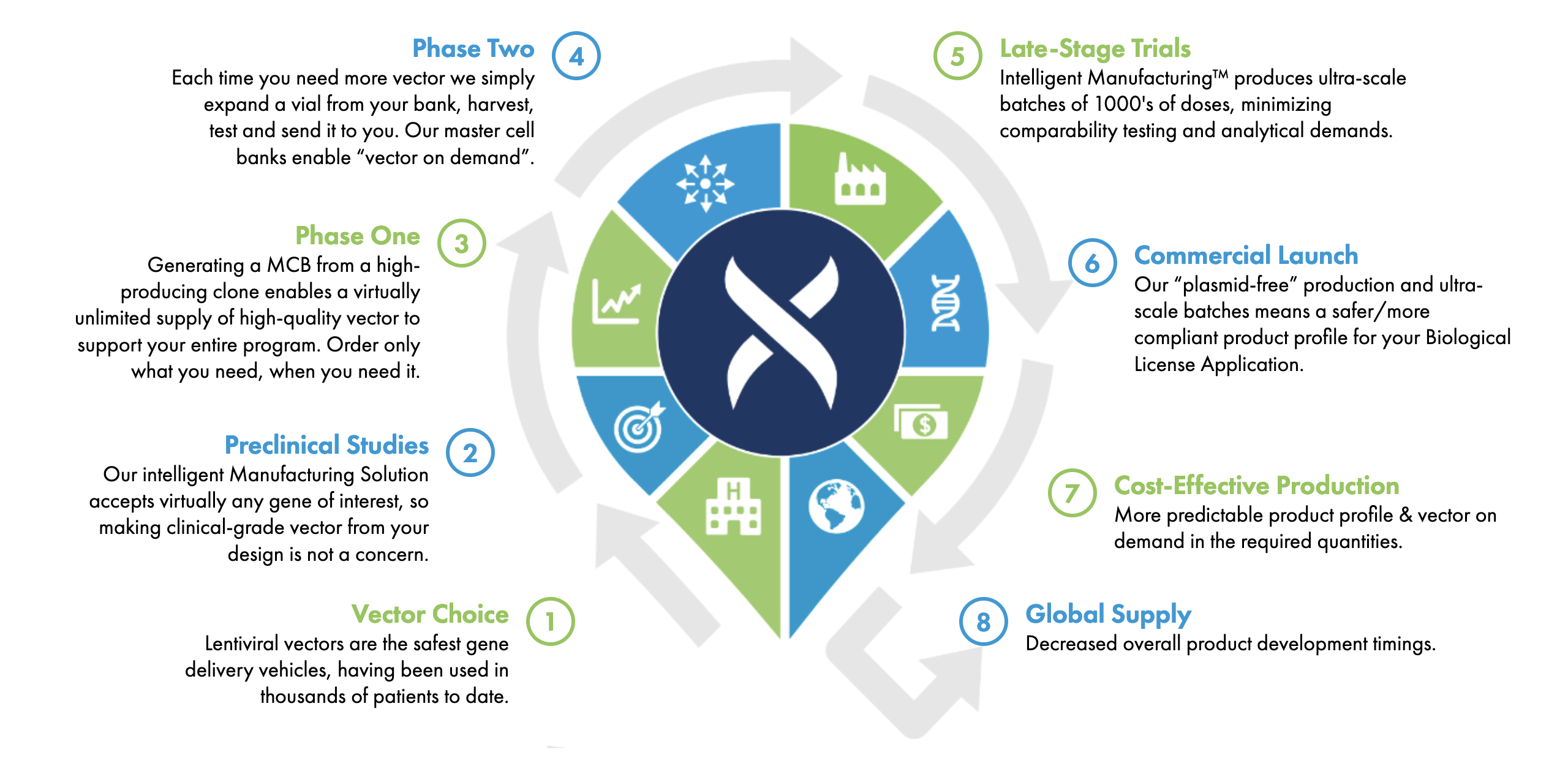
Our highly scalable platform supports your vector needs through drug development and commercial launch.
Phase Two
Each time you need more vector we simply expand a vial from your bank, harvest, test and send it to you. Our master cell banks enable “vector on demand”.
Phase One
Generating a MCB from a high- producing clone enables a virtually unlimited supply of high-quality vector to support your entire program. Order only what you need, when you need it.
Preclinical Studies
Our intelligent Manufacturing Solution accepts virtually any gene of interest, so making clinical-grade vector from your design is not a concern.
Vector Choice
Lentiviral vectors are the safest gene delivery vehicles, having been used in thousands of patients to date.

Late-Stage Trials
Intelligent Manufacturing™ produces ultra-scale batches of 1000's of doses, minimizing comparability testing and analytical demands.
Commercial Launch
Our “plasmid-free” production and ultra-scale batches means a safer/more compliant product profile for your Biological License Application.
Cost-Effective Production
More predictable product profile & vector on demand in the required quantities.
Global Supply
Decreased overall product development timings.
Vector Choice
Lentiviral vectors are the safest gene delivery vehicles, having been used in thousands of patients to date.
Preclinical Studies
Our intelligent Manufacturing Solution accepts virtually any gene of interest, so making clinical-grade vector from your design is not a concern.
Phase One
Generating a MCB from a high- producing clone enables a virtually unlimited supply of high-quality vector to support your entire program. Order only what you need, when you need it.
Phase Two
Each time you need more vector we simply expand a vial from your bank, harvest, test and send it to you. Our master cell banks enable “vector on demand”.
Late-Stage Trials
Intelligent Manufacturing™ produces ultra-scale batches of 1000's of doses, minimizing comparability testing and analytical demands.
Commercial Launch
Our “plasmid-free” production and ultra-scale batches means a safer/more compliant product profile for your Biological License Application.
Cost-Effective Production
More predictable product profile & vector on demand in the required quantities.
Global Supply
Decreased overall product development timings.
HOW CAN WE HELP?
CONTACT US TODAY.
iVexSol
17 Briden Street
Worcester, MA 01605

Want more information?
Or let us know where you are in your clinical development to learn more about how our solutions can help you now.



