
Pre-Clinical
Pre-clinical stage clients do not require clinical grade material for IND enabling and toxicity studies. Given the small scale of material needed to support pre-clinical studies one would not envision making a stable cell line, as transient material would be sufficient. We can support you with high quality technical-grade vector that will closely align with your Phase I product. We also offer packaging cell lines (currently available through an Early Access program) that provided the flexibility needed at early stages of clinical development.
How do you ensure your lentiviral vector is fit for purpose?
Vector Fitness Testing TM
If your vector is capable of high-titer production at lab scale, then iVexSol’s Intelligent Manufacturing will preserve and likely improve those titers. This is often however, not the case, and even at small scale, challenges such as splice variation, codon usage, and non-optimized promoter and backbone choices can rob your vector of titer and clinical efficacy.
iVexSol performs Vector Fitness Testing TM as part of our Intelligent Manufacturing to assure that you are delivering the best possible construct to your process.
How does iVexSol’s early-stage production differ from its competitors?
High Grade Material
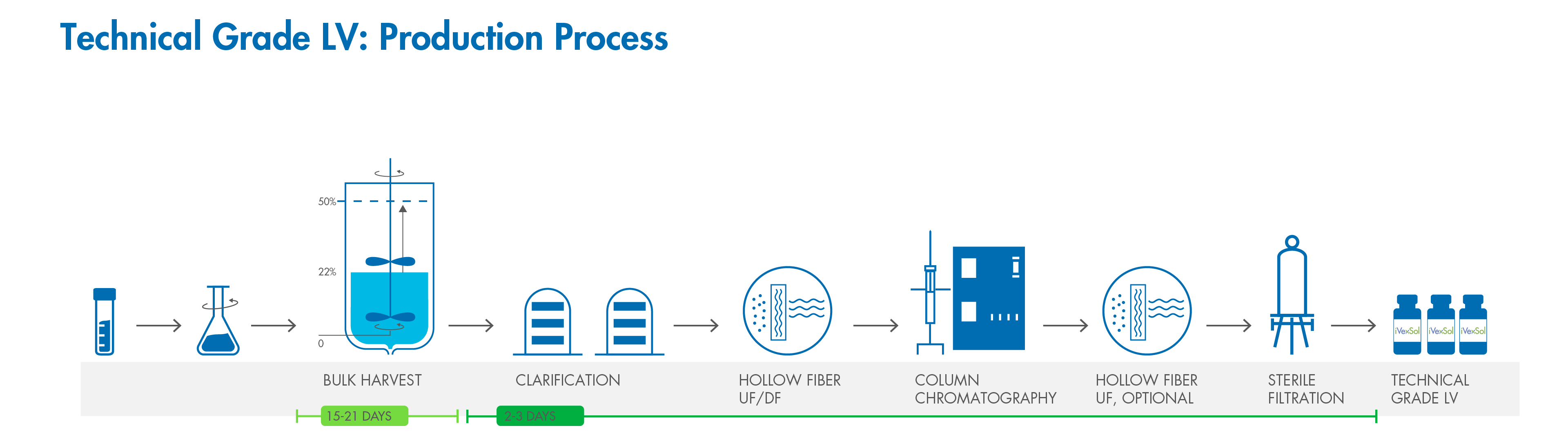
Unlike traditional technical grade purification methods, which relies solely on centrifugation, we purify and concentrate supernatants by sequential chromatographic steps, obtaining biologically active Lentiviral Vectors with an infectious titer and specific activity in the order of 108 -109 transducing unit (TU)/mL depending on the Gene of Interest (GOI).
Our purification workflow for transient transfection removes >99% of the starting plasmid, DNA, and protein impurities, resulting in a technical grade vector with high gene transfer efficiency.
Why is iVexSol producing transient material when we are a stable LVV producer?
Complete Lifecycle Support
During pre-clinical development where the safety and efficacy of the product is unknown, transient transfection offers significantly reduced development times and increased flexibility for Lentiviral Vector manufacturing compared to the generation of stable packaging and producer cell lines.
Once safety and efficacy are confirmed, we will start on the generation of stable cell lines that will support your clinical development and global launch.
If your vector is capable of high-titer production at lab scale, then iVexSol’s Intelligent Manufacturing will preserve and likely improve those titers. This is often however, not the case, and even at small scale, challenges such as splice variation, codon usage, and non-optimized promoter and backbone choices can rob your vector of titer and clinical efficacy.
iVexSol performs Vector Fitness Testing TM as part of our Intelligent Manufacturing to assure that you are delivering the best possible construct to your process.
Unlike traditional technical grade purification methods, which relies solely on centrifugation, we purify and concentrate supernatants by sequential chromatographic steps, obtaining biologically active Lentiviral Vectors with an infectious titer and specific activity in the order of 108 -109 transducing unit (TU)/mL depending on the Gene of Interest (GOI).
Our purification workflow for transient transfection removes >99% of the starting plasmid, DNA, and protein impurities, resulting in a technical grade vector with high gene transfer efficiency.
During pre-clinical development where the safety and efficacy of the product is unknown, transient transfection offers significantly reduced development times and increased flexibility for Lentiviral Vector manufacturing compared to the generation of stable packaging and producer cell lines.
Once safety and efficacy are confirmed, we will start on the generation of stable cell lines that will support your clinical development and global launch.
High Quality Technical-Grade Vector
iVexSol offers high quality technical-grade LVV produced by transient transfection which has been purified and concentrated by sequential chromatographic steps, resulting in infectious titers and specific activity in the order of 18 - 19 transducing unit (TU)/mL depending on the Gene of Interest (GOI). Our purification workflow for transient transfection removes >99% of the starting plasmid, DNA, and protein impurities, resulting in a vector prep that behaves much more like your future clinical grade preps resulting in less comparability testing and extended timelines. Fill out our RFI today in the chat bot to get your process started.
Start Your RFI Today
HOW CAN WE HELP?
CONTACT US TODAY.
iVexSol
17 Briden Street
Worcester, MA 01605


