
The Stable Lentiviral Vector Solution
iVexSol provides the transformative answer for lentiviral vector supply. Our next-generation, plasmid-free process delivers a variety of stable lentiviral vector solutions, including stable producer cell lines that save time and reduce costs and packaging cell lines that provide the flexibility needed for early stage CGTs. Our truly differentiated lentiviral vector technology opens up patient access to CAR-T and other advanced therapies.
Lentiviral vectors, though maintaining an outstanding safety record and being used in hundreds of clinical trials, have been limited by traditional production methods. The result: Long wait times, skyrocketing cost of goods, and chronic vector shortages.
What if there was a better way?
Meet iVexSol Intelligent ManufacturingTM
How does it work?
iVexSol’s next-generation stable lentiviral vector production platform begins with the generation of a clonally-derived Cell Bank of stable vector-producing cells for the production of your Gene of Interest. Here's how iVexSol Intelligent Vector SolutionsTM solves common LVV challenges.
Transduction
Serum-free, suspension host cells are engineered with microgram quantities of plasmid
Plasmid Engineering
Plasmids are linearized and re-engineered to include inducible constructs.
Vector Fitness Testing
We begin by fitness testing your current platform to ensure it is fit for purpose.
Selection
Cells are seeded, expanded, and screened in to identify highly productive clones.
Optimal Producer
Optimal producers are further screened for stability, functionality and safety.
MCB Generation
Optimal clones are expanded to create a Master Cell Bank.
Long Term Storage
MCB is cryopreserved and split 50/50 for off-site storage.
Vector Fitness Testing
We begin by fitness testing your current platform to ensure it is fit for purpose.
Plasmid Engineering
Plasmids are linearized and re-engineered to include inducible constructs.
Transduction
Serum-free, suspension host cells are engineered with microgram quantities of plasmid
Selection
Cells are seeded, expanded, and screened in to identify highly productive clones.
Optimal Producer
Optimal producers are further screened for stability, functionality and safety.
MCB Generation
Optimal clones are expanded to create a Master Cell Bank.
Long Term Storage
MCB is cryopreserved and split 50/50 for off-site storage.
Then, whether it be your initial production run or a subsequent run, each time you need more vector, all that's needed is to simply expand a vial from your bank, harvest and cryopreserve the precise amount of vector you require and then ship where needed. On demand: All within weeks, not months.
Vector On Demand TM
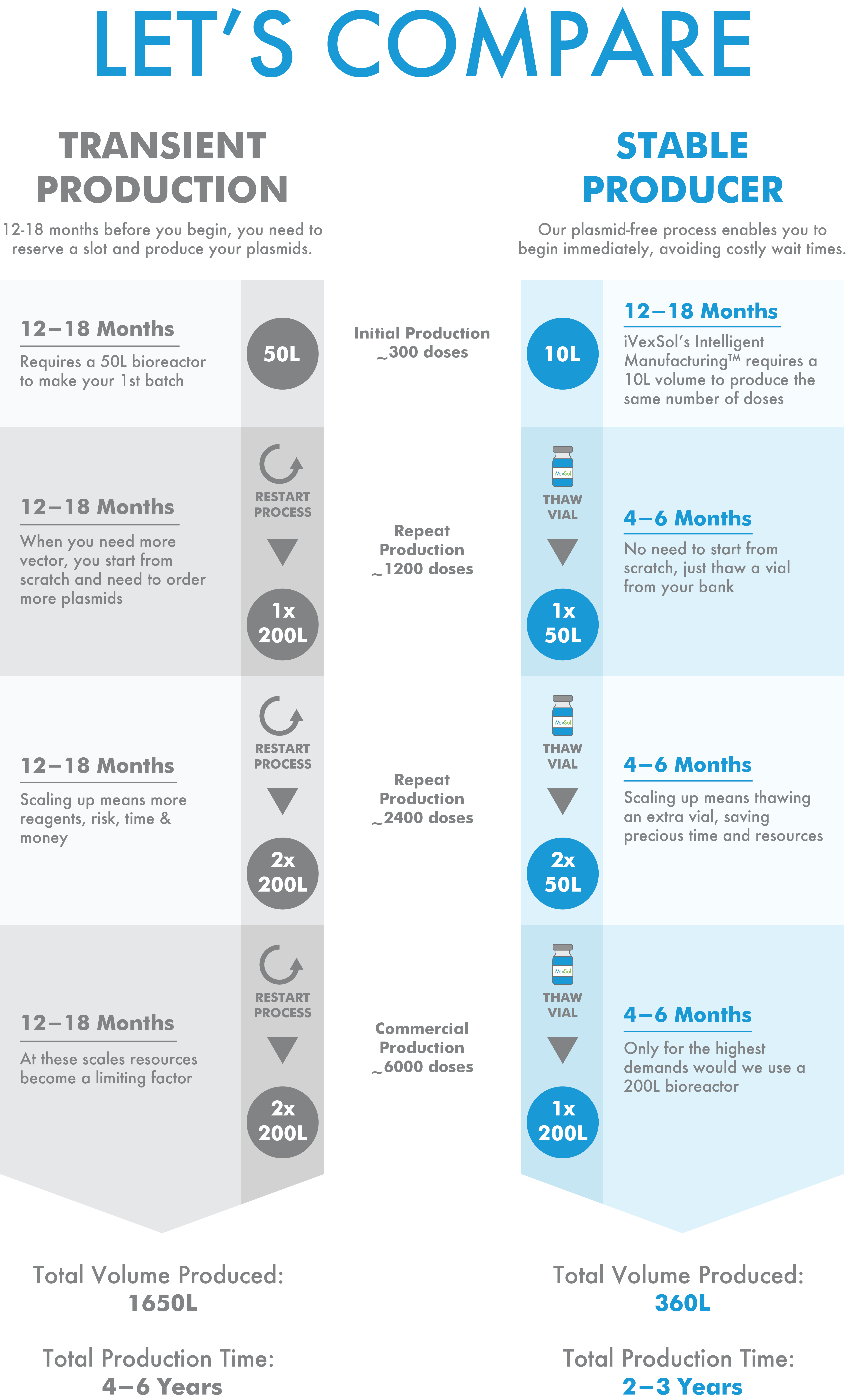
How does it impact my program?
iVexsol Intelligent ManufacturingTM ensures the rapid and reliable production of high quality, high-titer, ready to use vector on time and at any scale, accelerating your clinical development timeline.

Want more information?
Or let us know where you are in your clinical development to learn more about how our solutions can help you now.


